About Us

Inosan is a biotechnology company that has integrated innovation and internal development of knowledge and technology to manufacture medicines for vulnerable patients' emergencies.
Learn MoreOur strategy and products are aligned with the WHO plan that support global efforts to control, eliminate and eradicate neglected diseases
PRODUCTS
Not all products and/or indications are available in all territories
At Inosan Biopharma we develop and distribute the latest generation antivenoms that meet highest manufacturing standars
- Broad regional coverage of medically important species
- Highly purified
- High neutralizing capacity with low total protein content
- No preservatives
- Injectable lyophilizates
We manufacture state-of-the-art biotechnological medicines for the treatment of rare and neglected diseases
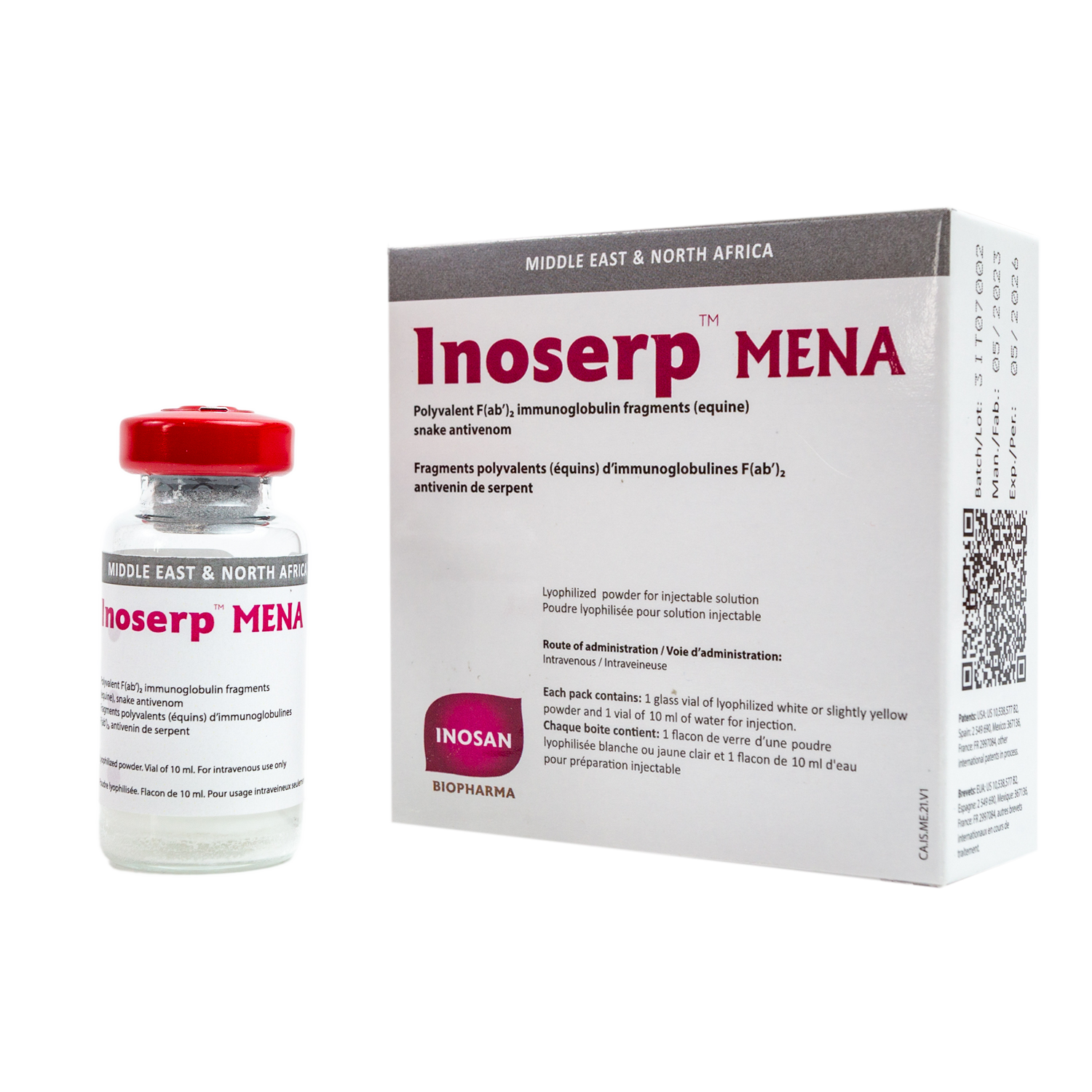
Is a polyvalent equine antivenom specifically indicated for the treatment of Sub- Middle East and North African snake bites.
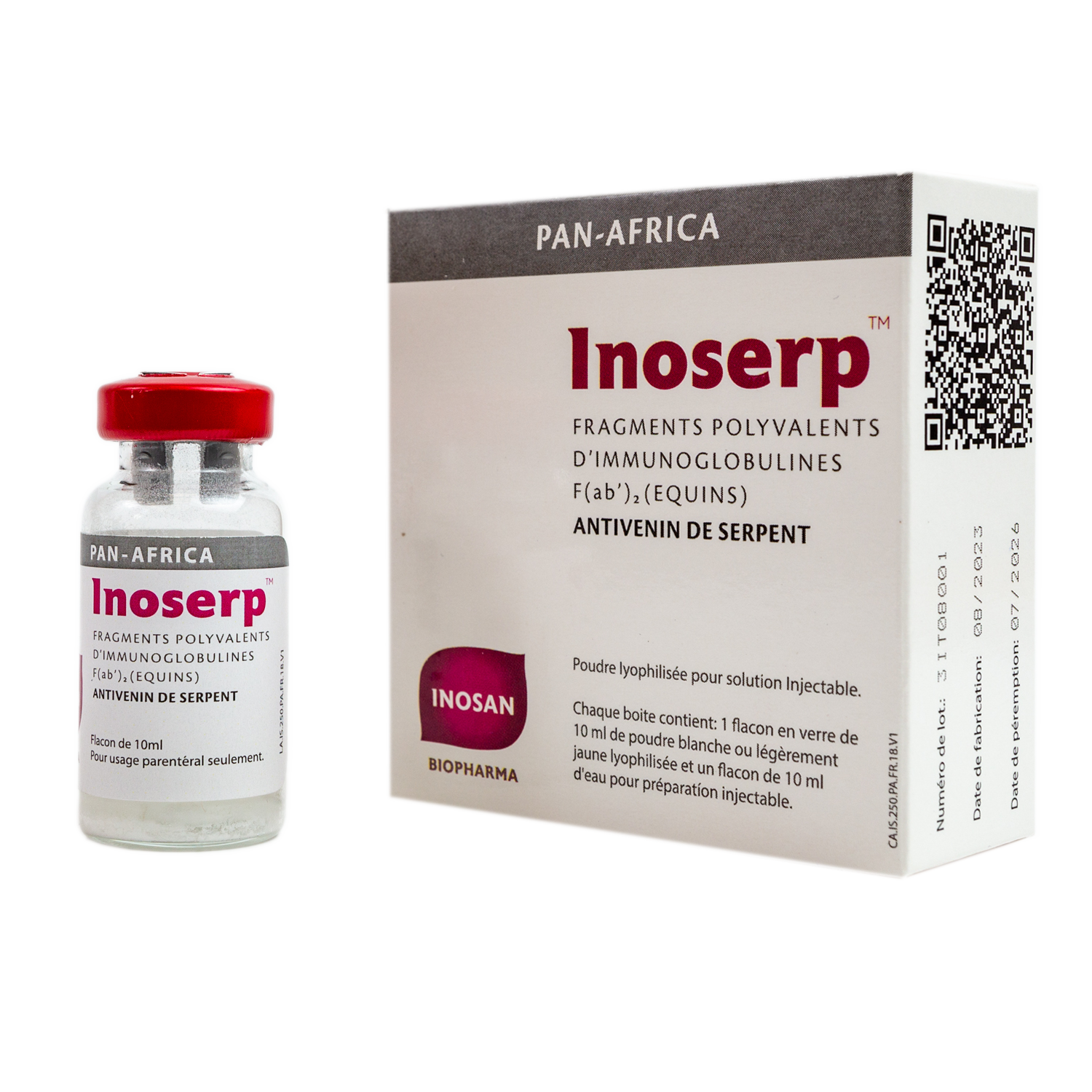
Is a polyvalent equine antivenom specifically indicated for the treatment of Sub-Saharan African snake bites.
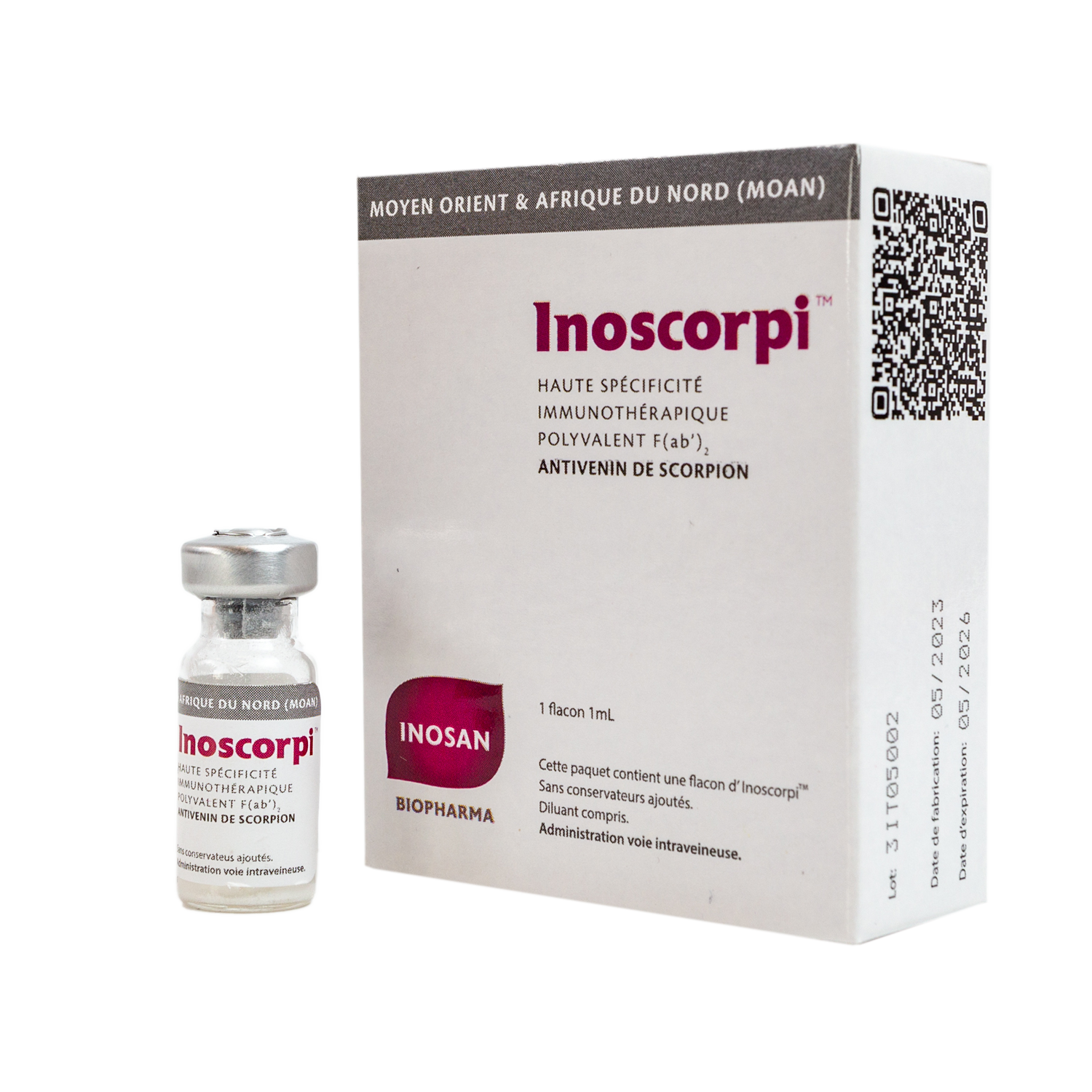
Is a polyvalent equine antivenom specifically indicated for the treatment of Middle East and North African scorpion stings.
More about inosan biopharma
At Inosan Biopharma we care about people's health and we go hand in hand with biotechnology to research and manufacture the latest generation of highly specific biological drugs.


Research & Development
Currently our scientists and researchers are developing new medicines for the treatment of rare and neglected diseases.

Events 2023
- IECT, July 14-28, Online
- Venoms & Toxins, August 22-24, Oxford (UK)
- IVECCS (Veterinary congress), Sep 08-12, Denver
- Denver Venom Conference, Sep 22, Denver
- Watamu snakebite conference, Sep 28-30, Watamu
- Pan American branch of IST congress, Nov 09-13, Brasilia
- Annual international NTD meeting or local Kenya event (Date TBD, Nairobi)
Events 2022
- Venom Week, July 18-21, Skysong Center, Scottsdale (Arizona)
- Venoms & Toxins, August 23-25, Oxford (UK)
- World congress of IST, Oct 16-21, Conrad Abu Dhabi Etihad Towers (United Arab Emirates)
FREQUENT MEDICAL QUESTIONS & ANSWERS
-
What is antivenom?
It is a biological therapy indicated for the treatment of poisoning by bites or stings of venomous or poisonous animals; for example, snakes, scorpions and spiders whose function is to counteract the effects of a venom or at least prevent the undesirable damage of the toxic compounds.
-
How are antivenoms classified?
Although there are several classifications according to their composition, purity and neutralizing capacity, these classifications include monovalent and polyvalent antivenoms.
Monovalent is the one that recognizes and neutralizes the venom of a single species and is due to the fact that in its production process only the venom of a single species was used.
Polyvalent, is one that recognizes and neutralizes the venom of at least two different species and its neutralizing capacity depends on the number of venoms of different species that were used in its production. -
How many types of antivenoms are there?
Complete serum
Complete IgG
Fab fragments
F(ab')2 fragments -
How many generations of antivenoms are there?
1st Generation Hyperimmune serum, biological product, low efficacy and safety.
2nd Generation Complete immunoglobulins (IgG). Serum, purified biological product of moderate efficacy and safety.
3rd and 4th Generation [Fab/ F(ab')2 antibody fragments]. Also called Fabotherapics, they are composed of the F(ab')2 fragment of the immunoglobulin, resulting from pepsin digestion of the antibodies can neutralize the venom and reduce hypersensitivity reactions of immediate type (anaphylaxis) and delayed type (serum sickness). Biotechnological product of high efficacy and safety. -
How is an antivenom manufactured?
1. The venom of an animal (snake, scorpion or spider) is selected.
2. Horses are injected with this venom(s) so that their immune system responds by generating antibodies to all the components of the venom(s).
3. Plasma is extracted from the horses' blood.
4. The hyperimmune plasma is subjected to purification processes and strict quality controls.
5. The result is a sterile solution of antibodies or fragments of them and they are packaged in liquid or powder form (lyophilized). -
What is the mechanism of action of antivenoms?
When the antivenom enters the organism it goes through 3 phases:
RECOGNITION: The F(ab')2 fragments specifically recognize the different toxic components of the venom.
PRECIPITATION: The F(ab')2 fragments bind to the toxic components of the venom to neutralize them, forming a complex (F(ab')2 fragment-toxic component).
ELIMINATION: The complex formed will facilitate the redistribution and elimination of the venom components from the organism. -
What is the specificity in antivenoms?
It is the ability of antibodies or antibody fragments to recognize and bind to the components of venoms, which correlates with their potency and efficacy. The higher the specificity, the higher the efficacy.
-
What are the main characteristics and properties of INOSAN antivenoms?
Inosan antivenoms are produced using a state-of-the-art biotechnology manufacturing platform that meets the highest international quality standards.
Effectively covers all major medically important species in the Sub-Saharan Africa, North Africa and Middle East regions.
High Safety and Efficacy profile measured in extensive clinical studies.
No cold chain dependency due to lyophilized formulation. -
How is the safety profile of INOSAN antivenoms determined?
By its level of highly purified F(ab')2 > 90%.
Because they have no preservatives.
Because they are pyrogen-free.
All of the above reduces the risk of allergic reactions and serum sickness. -
How is the efficacy profile of INOSAN antivenoms determined?
It is performed in animal models (mice), where the effective dose is determined according to the biological activity also known as "Neutralization Potency".
-
How is the regional coverage of INOSAN's antivenoms (AV´s) guaranteed?
The design of the INOSAN AV´s is based on the WHO recommendation (Category 1) of the species of major medical importance for each region (Sub-Saharan Africa and North Africa and Middle East).
-
What criteria must a reliable antivenom meet?
Effective: To cover most common or widespread clinically important venomous snakes that result in high levels of morbidity, disability or mortality.
Safety/Tolerability: To allow use in remote rural health facilities.
Stability: Important in tropical regions to facilitate distribution.
Accessibility: Reliable source of supply and sustained availability.
For more information, please contact: medinfo@inosanbiopharma.com
CONTACT
Spain
Arbea Business Campus, Building 2, Floor 2
Ctra. Fuencarral a Alcobedas km 3,8, 28108 Alcobendas (Madrid), Spain
(+34) 913 346031
Mexico
Lucerna 7, Col. Juárez
Mexico City, Mexico
info@inosanbiopharma.com
(+52) 55 51 40 1535
With representation in
USA, France, Ghana and Kenya
PHARMACOVIGILANCE

If you need to report an adverse event, please fill one of the attached FORMs (english or Français) and send it to:
pharmacovigilance@inosanbiopharma.com English
English
 Francais
Francais

