Inoserp Middle East & North Africa (MENA)
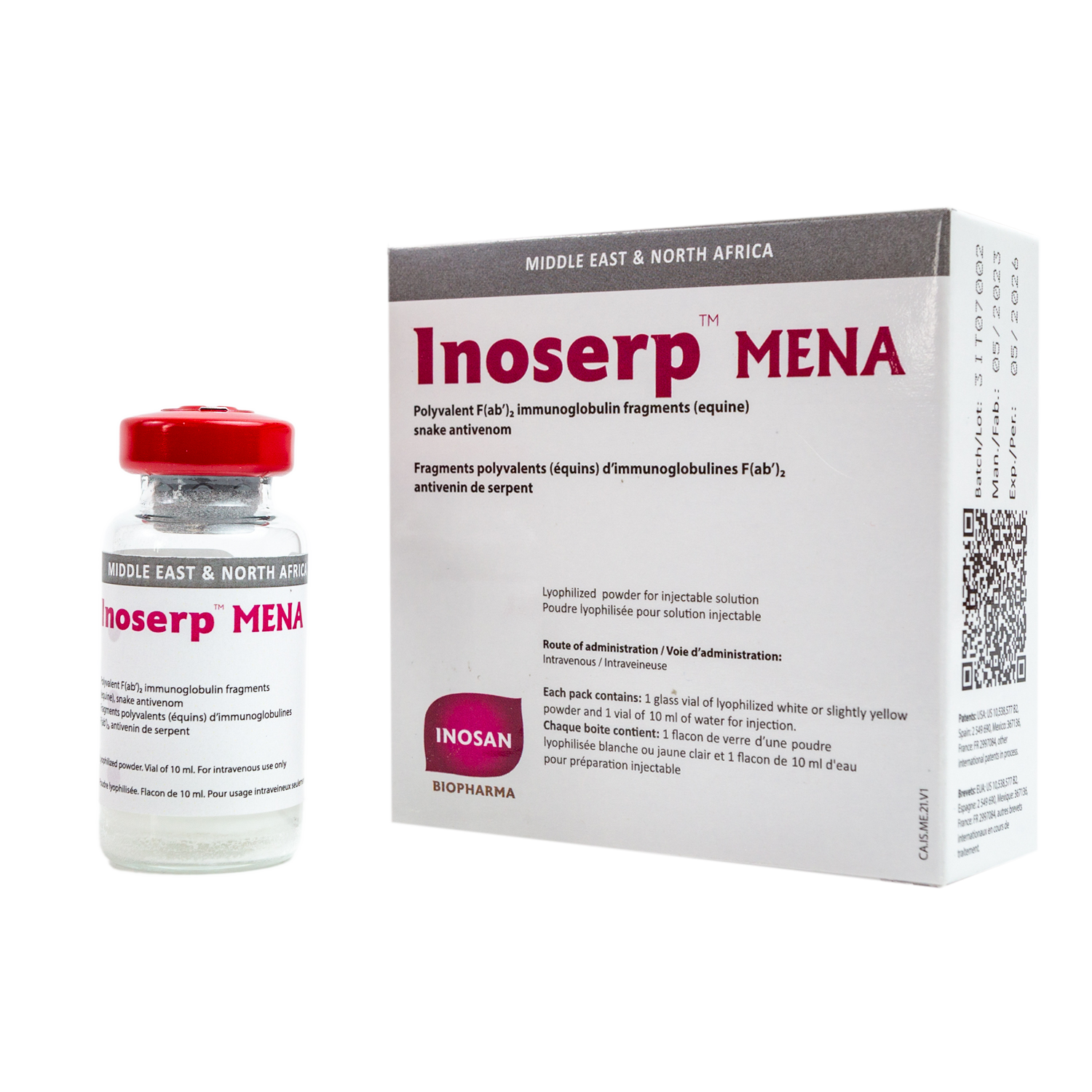
It is a sterile, highly purified lyophilized preparation of equine F(ab´)2 immunoglobulin fragments neutralizing with high specificity the venoms.
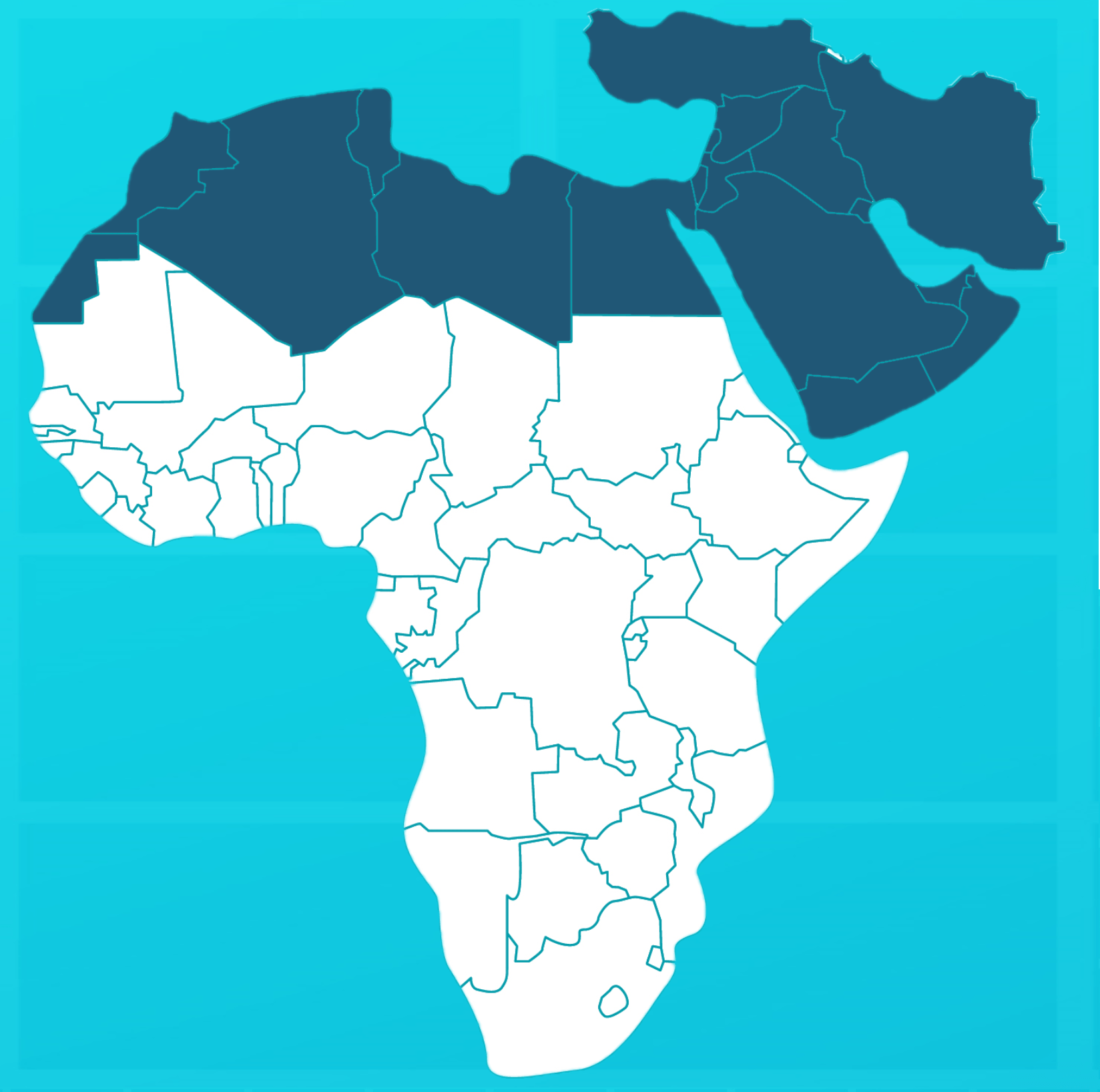
Region
Is a polyvalent equine antivenom specifically indicated for the treatment of sub- Middle East and North African snake bites.
Species
- Viperidae:
- Bitis arietans, Cerastes cerastes, Cerastes gasperettii, Cerastes vipera, Daboia deserti, Daboia mauritanica, Daboia palaestinae, Echis carinatus sochureki, Echis coloratus, Echis khosatzkii, Echis leucogaster, Echis megalocephalus, Echis omanensis, Echis pyramidum, Macrovipera lebetina obtusa, Macrovipera Iebetina transmediterranea, Macrovipera Iebetina turanica, Montivipera bornmuelleri, Montivipera raddei kurdistanica, Pseudocerastes fieldi, Pseudocerastes persicus, Vipera latastei.
- Elipidae:
- Naja haje, Naja nubiae, Naja oxiana, Naja pallida and Walterinnesia aegyptia.
FREQUENT MEDICAL QUESTIONS & ANSWERS
- What is the compositión of InoserpTM MENA?
InoserpTM MENA is made of antibodies specifically targeting the most dangerous species of the Middle East and North Africa (MENA) region. InoserpTM MENA is a polyvalent antivenom which means that it covers several species.
Read more
InoserpTM MENA is composed of equine derived antibodies or immunoglobulin fragments F(ab’)2, which have been raised against the venoms of medically important species of the MENA region.
-
What are the species covered by InoserpTM MENA?
InoserpTM MENA protects against the envenomation of the most dangerous snake species of the Middle East and North Africa (MENA) region. Overall, 27 medical important snake species both viperids and elapids are covered as follows:
Viperidae: Bitis arietans, Cerastes cerastes, Cerastes gasperettii, Cerastes vipera, Daboia deserti, Daboia mauritanica, Daboia palaestinae, Echis carinatus sochureki, Echis coloratus, Echis khosatzkii, Echis leucogaster, Echis megalocephalus, Echis omanensis, Echis pyramidum, Macrovipera lebetina obtusa, Macrovipera Iebetina transmediterranea, Macrovipera Iebetina turanica, Montivipera bornmuelleri, Montivipera raddei kurdistanica, Pseudocerastes fieldi, Pseudocerastes persicus, Vipera latastei.
Elapidae: Naja haje, Naja nubiae, Naja oxiana, Naja pallida and Walterinnesia aegyptia.
Read more
Overall, with 27 species, InoserpTM MENA provides the largest coverage of medically important species in MENA region.
-
What are the specificities of Inosan Biopharma antivenoms?
Inosan Biopharma antivenoms have been designed to offer the best protection against the most medical important snake species and also the best safety to patients thanks to a high quality manufacturing process.
Read more
- Inosan Biopharma antivenoms are polyvalent and cover most species that are medically important for the relevant region according to WHO guidelines. Venoms used for the immunization process come from species representative of the regions the antivenoms are intended for.
- Inosan antivenoms are made of highly specific F(ab’)2(1), meaning that there is a high proportion of effective F(ab’)2 capable to specifically bind to the toxic components of the venoms and neutralize them. Thanks to its high specificity, Inosan antivenoms can include more species in one vial with less proteins.
- Inosan Biopharma antivenoms are highly purified level with F(ab’)2 level reaching 95%(1). They have a low protein level (<10%, no more than 100 mg of protein per mL in the resuspended product) and are preservatives free.
-
What is the efficacy profile of InoserpTM MENA?
According to the clinical studies performed, InoserpTM MENA is effective in rapidly restoring hemostasis disorders and in reducing local complications(2)(3).
Read more
Retrospective data collected over 5 years (2016 - 2020) by the Moroccan poison center have been analyzed, indicating that Inoserp<TM MENA is effective in controlling thrombocytopenia in a large majority of patients(2).
-
What is the safety profile of InoserpTM MENA?
InoserpTM MENA has been used in 15 countries so far. The clinical experience indicates that InoserpTM MENA is very well tolerated. When occurring, adverse events are usually of mild intensity and resolve spontaneously or with treatment.
Read more
According to a retrospective analysis performed on 556 patients in Morocco, adverse events have been reported in less than 3% of patients(2). They may include sweating, nausea, rashes, modest decrease in blood pressure, anaphylactoid reaction (allergic type) associated with facial erythema and cough. The risk of an anaphylactic shock cannot be excluded but remains very rare. Similar reactions to serum sickness may appear after around 6 days of the onset of the treatment. The clinical signs are fever, pruritus, erythema or urticaria, adenopathy, and arthralgia. However, this type of reactions is observed in less than 1% of the patients.
-
What is the recommended dosage of InoserpTM MENA?
InoserpTM MENA should be administered as soon as possible after the first signs of envenomation in order to maximize its efficacy. The recommended dosage is 2 vials (through IV route). Further administration (2 vials) might be repeated, as necessary
Read more
InoserpTM MENA should be administered intravenously through slow direct injection of infusion. It is recommended to evaluate the patient every 2 hours after the initial administration and to renew administration (2 vials) as long as symptoms persist or are getting worse. A larger number of vials might be administered depending on the severity or the type of envenomation such as neurotoxic bites.
-
Are there any contraindications for the use of InoserpTM MENA?
People who are allergic to equine origin proteins or to excipients contained in the product should not be administered the antivenom.
Read more
Due to the fatal risk associated with envenomation, Inosan Biopharma antivenoms may be dispensed in the specific population of allergic patients to equine origin proteins, provided that treatment for anaphylactic shock can be implemented immediately.
-
Are there any specific recommendations for the use of InoserpTM MENA in children?
Dosage should be the same for adults and children, irrespective of weight, because the same quantity of venom is injected.
Read more
Envenomation in children leads to more severe symptoms and a higher rate of lethality and sequelae. This is due to their small body weight and volume that lead to a lower dilution of venom. A retrospective analysis on clinical data has shown that InoserpTM MENA was safe and effective in the pediatric population(3).
-
What are the the instructions for the storage of InoserpTM MENA?
Inosan Biopharma antivenoms are lyophilized and can be stored at room temperature up to 30°C with excursions up to 40°C for reduced periods.
Read more
Inosan Biopharma antivenoms are lyophilized, this makes the management of the antivenom very convenient and inexpensive compared to liquid presentations, particularly in tropical regions.
-
Is the manufacturing process of InoserpTM MENA GMP certified?
The manufacturing process of Inosan Biopharma antivenoms follows the highest GMP standards especially with regards to batch reproducibility and product stability.
Read more
Inosan Biopharma maintains GMP certification from COFEPRIS, the Mexican regulatory agency, recognized by the WHO, for the qualification of manufacturers of vaccines and biological products. In addition, Inosan follows the guidelines for the manufacturing of antivenom products established by the WHO.
For more information, please contact: medinfo@inosanbiopharma.com
References...
(1) Mathé et Al. A new generation of F(ab’)2 antivenoms made of highly purified and specific immunoglobulin fragments. Venoms Toxins 2020.
(2) Chafiq et Al. Données du centre anti poison et de pharmacovigilance du Maroc sur les morsures de serpents traitées entre 2015 et 2020 par l'antivenin Inoserp® MENA. STC 2022.
(3) Snakebite envenomation in children: An ongoing burden in Morocco. Essafti et Al. Annals of Medicine Surgery (2022)
